|
|
|
Yuri D. Ivanov, Ivan D. Shumov, Andrey F. Kozlov, Anastasia A. Valueva, Maria O. Ershova, Irina A. Ivanova, Alexander N. Ableev, Vadim Y. Tatur, Andrei A. Lukyanitsa, Nina D. Ivanov, Vadim S. Ziborov
1 Institute of Biomedical Chemistry, Moscow, Russia;
2 Joint Institute for High Temperatures of the Russian Academy of Sciences, Moscow, Russia
3 Foundation of Perspective Technologies and Novations, Moscow, Russia;
4 Faculty of Computational Mathematics and Cybernetics, Moscow State University, Moscow, Russia
5 Moscow State Academy of Veterinary Medicine and Biotechnology Named after Skryabin, Moscow, Russia
Abstract
Glycerol is employed as a functional component of heat-transfer fluids, which are of use in both bioreactors and various biosensor devices. At the same time, flowing glycerol was reported to cause considerable triboelectric effects. Herein, by using atomic force microscopy (AFM), we have revealed the long-term effect of glycerol flow, stopped in a ground-shielded coiled heat exchanger, on horseradish peroxidase (HRP) adsorption on mica. Namely, the solution of HRP was incubated in the vicinity of the side of the cylindrical coil with stopped glycerol flow, and then HRP was adsorbed from this solution onto a mica substrate. This incubation has been found to markedly increase the content of aggregated enzyme on mica—as compared with the control enzyme sample. We explain the phenomenon observed by the influence of triboelectrically induced electromagnetic fields of non-trivial topology. The results reported should be further considered in the development of flow-based heat exchangers of biosensors and bioreactors intended for operation with enzymes.
Keywords:
horseradish peroxidase; glycerol; atomic force microscopy; enzyme aggregation
1. Introduction
Enzymes have found numerous applications in biotechnology [1] and biomedical science [2]. In nature, enzymes catalyze reactions in living cells [3] and can be employed as catalysts in a wide range of commercially important processes [1]. The list of biotechnological applications of enzymes includes, for instance, food processing, synthesis of pharmaceuticals, paper fabrication, etc. [1,4]. As regards biomedicine, applications of enzymes in biosensors [2] and in diagnostic test kits [5,6] should be mentioned.
Enzyme-based catalysis requires the proper selection and careful maintenance of optimal process conditions since enzymes quickly lose their functional activity at extreme temperatures [7], pH values, ion concentrations, and pressures [8]. This is why biosensors and bioreactors intended for operation with enzymes are often equipped with thermal stabilization systems [9,10]. In these thermal stabilization systems, cylindrically wound pipes (or simply coils) with circulating heat-transfer fluid are often employed [11,12,13]. The use of glycerol as a component of heat-transfer fluids was shown to be promising [14,15]. At that, it should be emphasized that the flow of glycerol in a pipe induces electromagnetic fields due to the so-called triboelectric effect [16,17]. Electromagnetic fields generated upon the flow of glycerol can be quite strong [16], thus representing an important factor influencing the activity of enzymes [18]. The effect of a triboelectrically induced field on an enzyme can take place even after stopping the flow of heat transfer fluid [18].
One of the possible effects of electromagnetic fields on an enzyme is exhibited in the form of a change in its aggregation state upon adsorption onto a solid substrate surface [18,19,20]. This is an important point since surface-adsorbed enzymes are widely employed in biotechnology [1]. Aggregation of proteins, including enzyme proteins, is generally attributed to misfolding or partial unfolding of their polypeptide chains [21,22]. With regard to enzymes, their aggregation can also be related to a change in their hydration [18,19,23,24,25]. In general, aggregation is considered to cause a decrease in the functional activity of enzymes [26]. Colombie et al. demonstrated that inactivation of lysozyme in a bioreactor is accompanied by its aggregation [27]. On the other hand, Gentile et al. emphasized that aggregation of an enzyme can occur in the course of its functioning and does not inevitably imply activity loss [28]. Accordingly, enzyme aggregation and external factors influencing this process require further thorough investigation. In this respect, ultrasensitive methods such as atomic force microscopy (AFM) are of use [18,19,29,30,31] since they allow researchers to reveal even subtle effects of external impacts on enzyme aggregation [31].
Horseradish peroxidase (HRP) is a ~44 kDa enzyme glycoprotein [32,33]. It is widely employed as a component of enzyme-linked immunosorbent assay (ELISA) kits [34] and as a reporter enzyme in biosensors [35]. Furthermore, HRP has found many industrial applications in food technology [36], wastewater purification [37], and biofuel cell fabrication [38,39,40]. At that, the aggregation state of HRP was shown to be influenced by external magnetic [29,30] and electromagnetic fields [18,19,20]. Electromagnetic fields are ubiquitous in industry [41]. As mentioned above, the aggregation state of enzymes can influence their functional activity. This explains the importance of further in-depth investigation of the influence of electromagnetic fields on the aggregation state of HRP.
Our present study reveals a considerable 40 min after-effect (the so-called long-term effect [19]) of the glycerol flow in a cylindrically coiled heat exchanger on the aggregation state of HRP after incubation of its solution near the outer side of the coiled section, which has been covered with a grounded shield. In other words, the glycerol flow has been stopped prior to the incubation of the enzyme. The enzymatic activity of HRP has been found unaffected. Nevertheless, considerably increased content of aggregated enzyme has been revealed by AFM on the surface of mica substrates after the incubation of the enzyme in our experimental setup. Since the coil in the setup has been ground-shielded, the phenomenon observed can be explained by the occurrence of the so-called knotted electromagnetic fields [20].
2. Materials and Methods
2.1. Chemicals and Enzyme
Both the HRP enzyme (peroxidase from horseradish; cat. #6782) and its substrate 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS; cat. #A-1188) were purchased from Sigma (St. Louis, MO, USA). In AFM experiments, we used 2 mM Dulbecco’s modified phosphate buffered saline (PBSD buffer) was prepared by dissolving the salt mixture purchased from Pierce (USA) in the appropriate amount of ultrapure water. In spectrophotometry experiments, we used buffer salts and hydrogen peroxide purchased from Reakhim (Moscow, Russia). In all experiments, deionized ultrapure water (with 18.2 MΩ × cm resistivity), obtained with a Simplicity UV system (Millipore, Molsheim, France), was used.
The enzyme samples tested in the experiments represented 0.1 µM HRP solutions in 2 mM PBSD.
2.2. Experimental Setup and Enzyme Treatment
In general, the experimental setup was similar to that used in our previous study [19], but the enzyme was incubated near the heat exchanger’s coiled section. The location of the tested HRP solution (working sample) is schematically shown in Figure 1
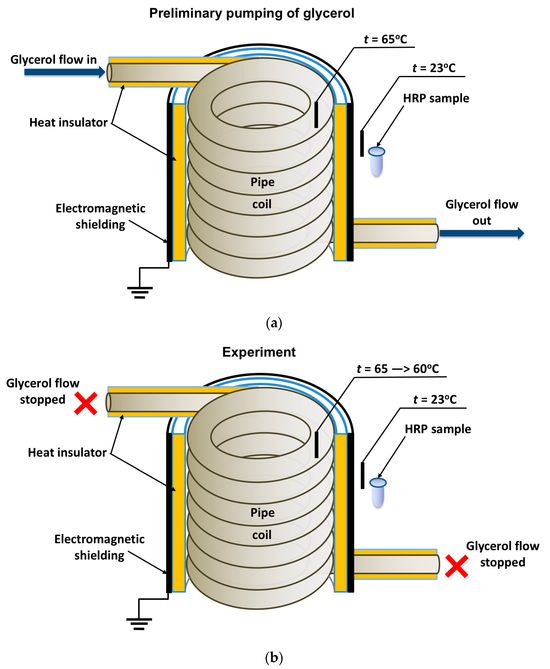
Figure 1. Schematic of the experimental setup illustrating the preliminary pumping of glycerol (a) and the incubation of the enzyme solution (working sample) near the coiled section of the heat exchanger after stopping the glycerol flow (b). Black rectangles indicate thermocouple-based thermometers used for temperature monitoring.
The cylindrically wound polymeric pipe modeled the heat exchanger. Prior to the experiment, warm (65 °C) glycerol (Glaconchemie GmbH, Merseburg, Germany) had been continuously pumped through this pipe at a flow rate of 9 L/s for 40 min. The glycerol temperature was monitored using an FY-10 digital thermometer, whose sensor was fixed on the inner side of the coil (Figure 1a,b). The use of warm glycerol provided the necessary fluidity of the glycerol and, hence, the desired flow rate [18,19].
Полный текст доступен в формате PDF (2659Кб)
Ivanov, Y.D.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ershova, M.O.; Ivanova, I.A.; Ableev, A.N.;vTatur, V.Y.; Lukyanitsa, A.A.; Ivanova, N.D.; et al. Atomic Force Microscopy Study of the Long-Term Effect of the Glycerol Flow, Stopped in a Coiled Heat Exchanger, on Horseradish Peroxidase. Micromachines 2024, 15 (4), 499; https://doi.org/10.3390/mi15040499
Yuri D. Ivanov, Ivan D. Shumov, Andrey F. Kozlov, Anastasia A. Valueva, Maria O. Ershova, Irina A. Ivanova, Alexander N. Ableev, Vadim Y. Tatur, Andrei A. Lukyanitsa, Nina D. Ivanov, Vadim S. Ziborov, Atomic Force Microscopy Study of the Long-Term Effect of the Glycerol Flow, Stopped in a Coiled Heat Exchanger, on Horseradish Peroxidase // «Академия Тринитаризма», М.,
|
|