|
|
|
Yuri D. Ivanov, Tatyana O. Pleshakova, Ivan D. Shumov, Andrey F. Kozlov, Anastasia A. Valueva, Irina A. Ivanova, Maria O. Ershova, Dmitry I. Larionov, Victor V. Repnikov, Nina D. Ivanova, Vadim Yu. Tatur, Igor N. Stepanov, Vadim S. Ziborov
1 Institute of Biomedical Chemistry, Moscow 119121, Russia
2 Bruker Ltd., Moscow 119017, Russia
3 Skryabin Moscow State Academy of Veterinary Medicine and Biotechnology, Moscow 109472, Russia
4 Foundation of Perspective Technologies and Novations, Moscow 115682, Russia
5 Joint Institute for High Temperatures of the Russian Academy of Sciences, Moscow 125412, Russia
Abstract
Atomic force microscopy (AFM)-based fishing is a promising method for the detection of low-abundant proteins. This method is based on the capturing of the target proteins from the analyzed solution onto a solid substrate, with subsequent counting of the captured protein molecules on the substrate surface by AFM. Protein adsorption onto the substrate surface represents one of the key factors determining the capturing efficiency. Accordingly, studying the factors influencing the protein adsorbability onto the substrate surface represents an actual direction in biomedical research. Herein, the influence of water motion in a flow-based system on the protein adsorbability and on its enzymatic activity has been studied with an example of horseradish peroxidase (HRP) enzyme by AFM, attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR) and conventional spectrophotometry. In the experiments, HRP solution was incubated in a setup modeling the flow section of a biosensor communication. The measuring cell with the protein solution was placed near a coiled silicone pipe, through which water was pumped. The adsorbability of the protein onto the surface of the mica substrate has been studied by AFM. It has been demonstrated that incubation of the HRP solution near the coiled silicone pipe with flowing water leads to an increase in its adsorbability onto mica. This is accompanied by a change in the enzyme’s secondary structure, as has been revealed by ATR-FTIR. At the same time, its enzymatic activity remains unchanged. The results reported herein can be useful in the development of models describing the influence of liquid flow on the properties of enzymes and other proteins. The latter is particularly important for the development of biosensors for biomedical applications—particularly for serological analysis, which is intended for the early diagnosis of various types of cancer and infectious diseases. Our results should also be taken into account in studies of the effects of protein aggregation on hemodynamics, which plays a key role in human body functioning.
Keywords: horseradish peroxidase; protein adsorption; protein aggregation; electromagnetic field; triboelectric effect
1. Introduction
Early diagnosis of oncological diseases in humans requires the detection of cancer-associated marker proteins at femtomolar (10−15M) or, better, even lower concentrations [1]. This is why the development of novel highly sensitive bioanalytical systems, which are able to overcome the 10−15M threshold, is an acute problem of modern biomedical research. Such highly sensitive systems are based on the so-called molecular detectors, capable of registering single target molecules [2,3]. In the majority of analytical systems, intended for the highly sensitive detection of proteins, a principle of molecular fishing is realized [3]. This principle is based on the capturing of the target biomolecules from the volume of a liquid sample onto the surface of a solid substrate, and the binding of these molecules with the substrate is registered with a detector. In particular, atomic force microscopy (AFM)-based molecular fishing allows one to detect proteins at ultra-low (down to 10−17M) concentrations [4]. The practical implementation of the AFM-based fishing approach comprises two main steps: (1) the incubation of the AFM substrate (the so-called AFM chip, onto which the target biomolecules are to be captured [4,5]) in the analyzed sample; and (2) counting of the captured target molecules on the substrate surface with an atomic force microscope. In [3], it was emphasized that reliable realization of the first step requires an efficient delivery of the target biomolecules from the sample solution onto the substrate surface. For this purpose, hydrodynamic intensification (that is, intensive stirring of the analyzed sample) of the delivery process is often used [3,4,6]. Nevertheless, despite the importance of the intensive delivery of the target biomolecules to the substrate surface, the overall possibility of their capturing is governed by their adsorbability onto this surface. The latter is determined by electrostatic and hydrophobic interactions between the biomolecules and the substrate surface. This is why the adsorption properties of proteins should be investigated.
In the development of bioanalytical systems, the following factor should also be considered. Since most proteins are heat-sensitive, thermal stabilization of measurement cells is required in such systems. This is often realized by employing flow-based heat exchangers [7], such as coils, where water is used as a heat-transfer agent. The flow of water through a coiled polymeric pipe was shown to induce an electromagnetic field, which can influence protein molecules, causing changes in their physicochemical properties [8]. Such a field, induced by liquid flow owing to a triboelectric effect, can extend to outside the coil. For this reason, it is interesting to study the influence of the flow-induced electromagnetic field on protein samples, incubated near a coiled pipe outside the coil.
Accordingly, we have studied the influence of an electromagnetic field, induced by the flow of water through a coiled polymeric pipe, on the adsorption properties of a protein, whose aqueous solution was incubated near the pipe outside the coil. Such a layout (Figure 1) can be realized when the protein fishing is performed simultaneously in a number of measuring cells. In control experiments, the measuring cell with the sample protein solution was placed far away, at a 10 m distance from the coil.
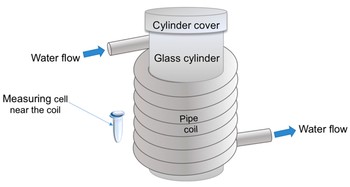
Figure 1. Schematic representation of the experimental setup employed for studying the effect of water flow on the properties of a protein. The measuring cell with the enzyme solution was placed either near the coil or far away. The silicone pipe is spiral-wound onto a glass cylinder to form a coil; water is pumped through the coiled pipe.
Herein, horseradish peroxidase (HRP) has been employed as a model protein. Its properties are well known, and this is why it has been employed in our experiments. HRP, which pertains to heme-containing enzymes, represents a glycoprotein, whose molecular weight makes up about 40 kDa to 44 kDa [9,10]. HRP catalyzes the oxidation of a wide range of both organic and inorganic compounds by hydrogen peroxide [11]. The HRP structure includes 77% α-helices and 12% β-sheets [12]. The HRP macromolecule includes 18% to 27% of carbohydrate chains, which stabilize the protein structure [10,13,14].
The adsorbability of HRP has been investigated by AFM using the direct surface adsorption method [15] of HRP from the sample solutions onto mica substrates, which are commonly employed in AFM studies [16]. AFM allows one to perform visualization of individual enzyme molecules [17]; this is of interest for single-molecule enzymology.
The physicochemical properties of HRP have been additionally studied by attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR). FTIR is commonly employed to study protein secondary structures [18,19,20] and protein–protein interactions [21,22]. This method represents a useful tool in monitoring changes in protein secondary structure within the range of Amide I and Amide II bands, from 1700 to 1500 cm−1 [23]. ATR-FTIR finds its application in studying proteins in insoluble and aggregated states [24].
In parallel, the enzymatic activity of HRP has been monitored by conventional spectrophotometry. In our research, these three methods have been employed together to study the influence of a flow-induced electromagnetic field on the properties of the HRP enzyme protein.
Herein, we used a spiral-wound (coiled) silicone pipe modeling a biosensor’s flow section (see Figure 1). A standard Eppendorf-type polypropylene test tube, modeling a biosensor’s measuring cell, placed near and outside the coil, contained the test solution of the HRP model protein (Figure 1). By AFM, an increased adsorbability of the HRP protein structures has been revealed after the incubation of the HRP solution near the coil with flowing water. Moreover, a change in the mutual intensity of Amide II to Amide I has been observed in the ATR-FTIR spectra. This indicates a change in the HRP secondary structure, occurring during its incubation near the coil. At that, no change in the enzymatic activity of HRP has been observed.
The results obtained herein can be useful in biosensor-based studies of the structure of proteins and their complexes. Our results can also be used in the development of serological methods for the early diagnosis of diseases (such as brain cancer, prostate cancer, etc.) in humans. These data can also be of use in studying hemodynamics in the human body.
Полный текст доступен в формате PDF (2637Кб)
Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Larionov, D.I.; Repnikov, V.V.; Ivanova, N.D.; Tatur, V.Y..; Stepanov, I.N.; Ziborov, V.S. AFM and FTIR Investigation of the Effect of Water Flow on Horseradish Peroxidase. Molecules 2021, 26(2), 306. https://doi.org/10.3390/molecules26020306 (registering DOI)
Yuri D. Ivanov, Tatyana O. Pleshakova, Ivan D. Shumov, Andrey F. Kozlov, Anastasia A. Valueva, Irina A. Ivanova, Maria O. Ershova, Dmitry I. Larionov, Victor V. Repnikov, Nina D. Ivanova, Vadim Yu. Tatur, Igor N. Stepanov, Vadim S. Ziborov, AFM and FTIR Investigation of the Effect of Water Flow on Horseradish Peroxidase // «Академия Тринитаризма», М.,
|
|